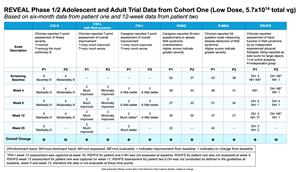
Data from first adult patient in REVEAL Phase 1/2 trial showed TSHA-102 (low dose, 5.7x1014 total vg) was well-tolerated with no treatment-emergent SAEs as of 35-week assessment, with sustained improvement across key efficacy measures at decreased steroid levels and new improvement in RSBQ at month six
Data from second adult patient showed TSHA-102 (low dose, 5.7x1014 total vg) was well-tolerated with no treatment-emergent SAEs as of 19-week assessment, with sustained improvement across key efficacy measures, significantly reduced seizure events and new improvement in R-MBA at week 12
Principal Investigator observed sustained and new improvements across multiple clinical domains following completion of steroid taper for patient one through 35-weeks post-treatment and for patient two through 19-weeks post-treatment at decreased steroid levels
Received Independent Data Monitoring Committee approval of Company’s request to proceed to early advancement to cohort two (high dose, 1x1015 total vg) in REVEAL adolescent and adult trial, and approval to dose second pediatric patient in cohort one (low dose, 5.7x1014 total vg) in REVEAL pediatric trial
Initial data from cohort one (low dose, 5.7x1014 total vg) in REVEAL pediatric trial expected mid-2024;
initial data from cohort two (high dose, 1x1015 total vg) in both trials (adolescent/adult and pediatric) expected in 2H 2024
Conference call and live webcast today at 4:30 PM Eastern Time
“We are highly encouraged by the safety profile and durable response reported in the longer-term data from the low dose cohort in our REVEAL adolescent and adult trial. Importantly, following completion of the steroid taper for the first patient and at decreased steroid levels for the second patient, both patients showed sustained improvements across multiple clinical domains, as well as new improvements compared to earlier post-treatment assessments, which supports the transformative potential of TSHA-102,” said
Dr.
Data Summary from Cohort One (Low Dose, 5.7x1014 total vg) of the REVEAL Phase 1/2 Adolescent and Adult Trial
TSHA-102 in Rett syndrome: a self-complementary intrathecally delivered AAV9 gene transfer therapy in clinical evaluation for Rett syndrome, a rare genetic neurodevelopmental disorder caused by mutations in the X-linked MECP2 gene. TSHA-102 utilizes a novel miRARE technology designed to mediate levels of MECP2 in the CNS on a cell-by-cell basis without risk of overexpression. The safety and preliminary efficacy of TSHA-102 are being evaluated in female patients aged 12-years and older with Rett syndrome due to MECP2 loss-of-function mutation in the REVEAL Phase 1/2 adolescent and adult trial, a first-in-human, open-label, randomized, dose-escalation and dose-expansion study taking place in
Results from the first patient (large MECP2 deletion; associated with severe phenotype) and second patient (missense MECP2 mutation; associated with milder phenotype) with late motor deterioration stage four Rett syndrome dosed with TSHA-102 in the low dose cohort (5.7x1014 total vg):
- Generally well-tolerated with no treatment-emergent serious adverse events (SAEs) as of 35-week assessment post-treatment for patient one and 19-week assessment post-treatment for patient two
- Sustained and new improvements observed across multiple clinical domains, as of 35-weeks post-treatment for patient one following completion of steroid taper at week 33, and 19-weeks post-treatment for patient two at decreased steroid levels (taper began at week 17), based on clinical observations by the Principal Investigator (PI), including:
- Autonomic function: improved breathing patterns, sleep quality/duration and circulation (patient one), and improved breathing patterns and circulation (patient two)
- Socialization/Communication: improved social interest, vocalization and ability to use eye-driven communication device (patient one), and improved social interest (patient two)
- Motor skills: improved hand function and gained ability to sit unassisted and move legs (patient one), and improved hand stereotypies (patient two)
- Seizures: stable seizure events (patient one), and significantly reduced seizure events (patient two)
- Seizure Diary showed stable seizure events at lower levels of anti-seizure medication relative to baseline through 35-weeks post-treatment in patient one, and significantly reduced seizure events with lower levels of anti-seizure medication relative to baseline through 19-weeks post-treatment in patient two, based on caregiver-reported medical history
- Clinical improvements seen across key efficacy measures in both patients include:
- Patient one at six-month assessment: Sustained improvement in Clinical Global Impression–Improvement (CGI-I), Clinical Global Impression–Severity (CGI-S), Parental Global Impressions–Improvement (PGI-I), Revised Motor Behavior Assessment (R-MBA), Rett Syndrome Hand Function Scale (RSHFS) and Seizure Diaries, at decreased steroid levels, with new improvement in Rett Syndrome Behavior Questionnaire (RSBQ)
- Patient two at 12-week assessment: Sustained improvement in CGI-I, PGI-I and RSBQ, with new improvement in R-MBA and Seizure Diaries
- Figure accompanying this announcement is available here.
Recent Corporate and Program Highlights
- Strengthened clinical and regulatory leadership with the promotion of
Meredith Schultz , M.D., M.S., to Chief Medical Officer andRumana Haque-Ahmed to Chief Regulatory Officer, reporting toSukumar Nagendran , M.D., President and Head of R&D.Dr. Schultz is a board-certified, licensed pediatric neurologist experienced in treating patients with Rett syndrome and leading gene therapy clinical trials. She brings more than 17 years of clinical experience and will lead the Company’s clinical development, clinical operations, medical affairs and safety activities.Rumana Haque-Ahmed brings nearly 30 years of experience in regulatory strategy and product development in the biopharmaceutical space. She will continue to lead the Company’s regulatory affairs department and initiatives. - REVEAL Phase 1/2 Adolescent and Adult Trial (
Canada andU.S. ):- Completed dosing in cohort one (low dose, n=2) of 5.7x1014 total vg.
- Received IDMC approval of the Company’s request to dose escalate immediately, enabling early advancement to cohort two (high dose, n=3) of 1x1015 total vg.
- Announced expansion of ongoing trial in
Canada into theU.S. and initiated site activation.
- REVEAL Phase 1/2 Pediatric Trial (
U.S. andUnited Kingdom (U.K. )):- Received IDMC approval to dose the second pediatric patient in cohort one (low dose, n=3) of 5.7x1014 total vg following review of initial clinical data from the six-week post-treatment assessment.
- Received authorization from
U.K. Medicines and Healthcare Products Regulatory Agency (MHRA) of Clinical Trial Application (CTA) for TSHA-102, enabling expansion of ongoing trial inU.S. into theU.K. Received Innovative Licensing and Access Pathway (ILAP) designation for TSHA-102 fromU.K. MHRA. The ILAP aims to facilitate patient access to novel treatments by accelerating time to market through opportunities for enhanced engagements withU.K. regulatory authorities and other stakeholders.
Anticipated 2024 Milestones
- REVEAL Adolescent and Adult Trial
- Dosing of the first patient in cohort two (high dose, n=3) of 1x1015 total vg expected in the second quarter of 2024.
- Initial safety and efficacy data from cohort two expected in the second half of 2024.
- REVEAL Pediatric Trial
- Dosing of the second patient in cohort one (low dose, n=3) of 5.7x1014 total vg expected in the first quarter of 2024.
- Initial safety and efficacy data from cohort one expected in mid-2024.
- Initial safety and efficacy data from cohort two (high dose, n=3) of 1x1015 total vg expected in the second half of 2024.
Full-Year 2023 Financial Highlights
Revenue: Revenue for the full year ended
Research and Development Expenses: Research and development expenses were
General and Administrative Expenses: General and administrative expenses were
Net loss: Net loss for the full year ended
Cash and cash equivalents: As of
Conference Call and Webcast Information
Taysha management will hold a conference call and webcast today at 4:30 p.m. ET to review its financial and operating results and to provide corporate and clinical updates. The dial-in number for the conference call is 877-407-0792 (
About TSHA-102
TSHA-102 is a self-complementary intrathecally delivered AAV9 investigational gene transfer therapy in clinical evaluation for Rett syndrome. Designed as a one-time treatment, TSHA-102 aims to address the genetic root cause of the disease by delivering a functional form of MECP2 to cells in the CNS. TSHA-102 utilizes a novel miRNA-Responsive Auto-Regulatory Element (miRARE) technology designed to mediate levels of MECP2 in the CNS on a cell-by-cell basis without risk of overexpression. TSHA-102 has received Fast Track designation and Orphan Drug and Rare Pediatric Disease designations from the FDA and has been granted Orphan Drug designation from the
About Rett Syndrome
Rett syndrome is a rare neurodevelopmental disorder caused by mutations in the X-linked MECP2 gene encoding methyl CpG-binding protein 2 (MeCP2), which is essential for regulating neuronal and synaptic function in the brain. The disorder is characterized by loss of communication and hand function, slowing and/or regression of development, motor and respiratory impairment, seizures, intellectual disabilities and shortened life expectancy. Rett syndrome progression is divided into four key stages, beginning with early onset stagnation at 6 to 18 months of age followed by rapid regression, plateau and late motor deterioration. Rett syndrome primarily occurs in females and is one of the most common genetic causes of severe intellectual disability. Currently, there are no approved disease-modifying therapies that treat the genetic root cause of the disease. Rett syndrome caused by a pathogenic/likely pathogenic MECP2 mutation is estimated to affect between 15,000 and 20,000 patients in the
About
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as “anticipates,” “believes,” “expects,” “intends,” “projects,” “plans,” and “future” or similar expressions are intended to identify forward-looking statements. Forward-looking statements include statements concerning the potential of TSHA-102, including the reproducibility and durability of any favorable results initially seen in patient dosed to date in clinical trials, and our other product candidates, to positively impact quality of life and alter the course of disease in the patients we seek to treat, our research, development and regulatory plans for our product candidates, including the timing of initiating additional trials and reporting data from our clinical trials, the potential for these product candidates to receive regulatory approval from the FDA or equivalent foreign regulatory agencies, and our current cash resources supporting our planned operating expenses and capital requirements into 2026. Forward-looking statements are based on management’s current expectations and are subject to various risks and uncertainties that could cause actual results to differ materially and adversely from those expressed or implied by such forward-looking statements. Accordingly, these forward-looking statements do not constitute guarantees of future performance, and you are cautioned not to place undue reliance on these forward-looking statements. Risks regarding our business are described in detail in our
Condensed Consolidated Statements of Operations (in thousands, except share and per share data) |
|||||||
| For the Year Ended |
|||||||
| 2023 | 2022 | ||||||
| Revenue | $ | 15,451 | $ | 2,502 | |||
| Operating expenses: | |||||||
| Research and development | 56,778 | 91,169 | |||||
| General and administrative | 30,047 | 37,360 | |||||
| Impairment of long-lived assets | 1,065 | 36,420 | |||||
| Total operating expenses | 87,890 | 164,949 | |||||
| Loss from operations | (72,439 | ) | (162,447 | ) | |||
| Other income (expense): | |||||||
| Change in fair value of warrant liability | (34,718 | ) | — | ||||
| Loss on debt extinguishment | (1,398 | ) | — | ||||
| Change in fair value of term loan | (1,538 | ) | — | ||||
| Interest income | 3,572 | 249 | |||||
| Interest expense | (4,998 | ) | (3,798 | ) | |||
| Other expense | (47 | ) | (18 | ) | |||
| Total other expense, net | (39,127 | ) | (3,567 | ) | |||
| Net loss | $ | (111,566 | ) | $ | (166,014 | ) | |
| Net loss per common share, basic and diluted | $ | (0.96 | ) | $ | (3.78 | ) | |
| Weighted average common shares outstanding, basic and diluted | 116,121,482 | 43,952,015 | |||||
Condensed Consolidated Balance Sheet Data (in thousands, except share and per share data) |
|||||||
2023 |
2022 |
||||||
| ASSETS | |||||||
| Current assets: | |||||||
| Cash and cash equivalents | $ | 143,940 | $ | 87,880 | |||
| Restricted cash | 449 | — | |||||
| Prepaid expenses and other current assets | 3,479 | 8,537 | |||||
| Assets held for sale | 2,000 | — | |||||
| Total current assets | 149,868 | 96,417 | |||||
| Restricted cash | 2,151 | 2,637 | |||||
| Property, plant and equipment, net | 10,826 | 14,963 | |||||
| Operating lease right-of-use assets | 9,582 | 10,943 | |||||
| Other non-current assets | 304 | 1,316 | |||||
| Total assets | $ | 172,731 | $ | 126,276 | |||
| LIABILITIES AND STOCKHOLDERS' EQUITY | |||||||
| Current liabilities: | |||||||
| Accounts payable | $ | 6,366 | $ | 10,946 | |||
| Accrued expenses and other current liabilities | 12,284 | 18,287 | |||||
| Deferred revenue | 18,106 | 33,557 | |||||
| Total current liabilities | 36,756 | 62,790 | |||||
| Term loan, net | 40,508 | 37,967 | |||||
| Operating lease liability, net of current portion | 18,953 | 20,440 | |||||
| Other non-current liabilities | 1,577 | 4,130 | |||||
| Total liabilities | 97,794 | 125,327 | |||||
| Stockholders' equity | |||||||
| Preferred stock, |
— | — | |||||
| Common stock, |
2 | 1 | |||||
| Additional paid-in capital | 587,942 | 402,389 | |||||
| Accumulated deficit | (513,007 | ) | (401,441 | ) | |||
| Total stockholders’ equity | 74,937 | 949 | |||||
| Total liabilities and stockholders' equity | $ | 172,731 | $ | 126,276 | |||
Company Contact:
Director, Head of Corporate Communications and Investor Relations
hcollins@tayshagtx.com
Media Contact:
Inizio Evoke
Carolyn.hawley@inizioevoke.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/466835b0-a81c-400a-af61-1395051604ec

Source: Taysha Gene Therapies, Inc.